Following Major Acquisition, TekniPlex Healthcare to Showcase Expanded Interventional Capabilities at MD&M West
- Published: January 18, 2024
Now a full-fledged CDMO, TekniPlex Healthcare to emphasize concept-to-completion portfolio at two booths.
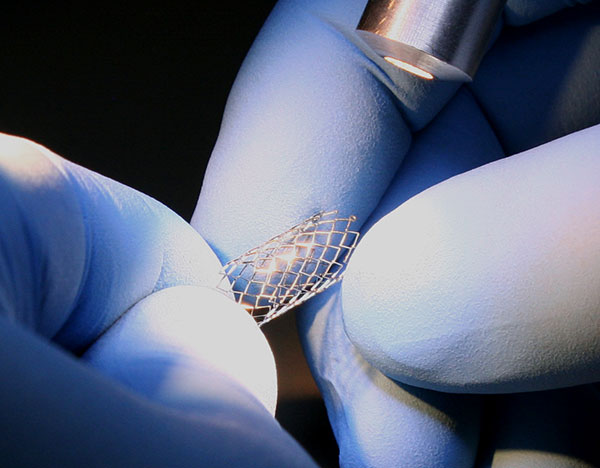
TekniPlex Healthcare, which utilizes advanced materials science expertise to help deliver better patient outcomes, will display its enhanced and expanded set of interventional capabilities with a two-booth presence at Medical Design & Manufacturing West, February 6-8 in Anaheim, CA. Following its December acquisition of Seisa Medical, the TekniPlex Healthcare’s MD&M footprint comprises both its own branded Booth #3227, as well as Seisa’s Booth #2266.
The acquisition makes TekniPlex Healthcare a fully capable CDMO, broadening and bolstering its materials science and process technologies for minimally invasive and interventional therapy devices. Combined with its diverse legacy portfolio, TekniPlex Healthcare can now co-develop critical components and sub-assemblies, and provide engineering services for comprehensive device design. This translates to end-to-end capabilities for Class II & Class III medical devices, with an emphasis on catheter-based and single-use sterile devices.
“TekniPlex now stands as a truly concept-to-completion partner for minimally invasive delivery systems and implantable devices – a full-fledged CDMO,” said Chris Qualters, CEO of TekniPlex Healthcare. “Our interventional products capabilities span everything from design, material selection, molding and extrusions, stents and balloons to full contract assembly and healthcare packaging services for medical devices.”
TekniPlex Healthcare also will be highlighting its antimicrobial medical compounds, which can be utilized to produce patient-contact products including catheters, connectors, gas hoses, components of injection systems such as syringes, and pens. The products can incorporate silver ion technology proven to drastically reduce contamination risk from bacteria buildup; unlike disinfectants that last just a few hours, formulating a medical product with antimicrobial compounds provides protection throughout the product's lifespan. Currently, TekniPlex Healthcare integrates the silver ion additives – which have natural antimicrobial properties – with its broad array of current medical grade flexible and rigid PVC compounds, or its medical grade CELLENE® TPE compounds.
Another offering will be the company’s range of solvent-bondable thermoplastic elastomer (TPE) tubing produced with TekniPlex Healthcare’s proprietary compound formulations. An alternative to polyvinyl chloride (PVC), the product offering is designed to bridge the PVC-alternative tubing ease-of -bonding technology gap. Free of silicone, latex, phthalate and halogen, they are suitable for a wide variety of medical device tubing applications.
The company also will be highlighting its medical device packaging offerings, including a variety of grid lacquer coated paper specifications and coated Tyvek® options. Later this year, TekniPlex Healthcare will be opening a new facility in Madison, WI, significantly boosting its lamination capacity for a broad range of materials including PET, nylon, paper, foil, PE, EAA and ionomer, in both peelable and non-peelable structures. The plant’s new coating and lamination assets will increase capacity for an array of products such as coated Tyvek® and reinforced papers.
For more information visit www.tekni-plex.com/healthcare.