At Pharmapack Paris, Schreiner MediPharm to Showcase New First-Opening Indication Feature for Protective Needle-Trap Solution
- Published: January 11, 2023
Novel combination of needlestick and product protection will debut at Hall 7.2, Booth D46
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, will introduce a new first-opening indication for its popular Needle-Trap solution at Pharmapack Paris, February 1-2. At Booth D46 in Hall 7.2, the company will debut a special security seal integrated into Needle-Trap that reliably indicates whether a prefilled syringe has been previously opened, helping protect the integrity of its contents until final use.
Drug packaging integrity is a critical aspect of a secure supply chain for pharmaceutical manufacturers. Regulations such as the EU Falsified Medicines Directive contribute to supply chain security, but generally address only secondary packaging. As a result, primary containers like prefilled syringes require more specific solutions for tamper evidence and first-opening indication.
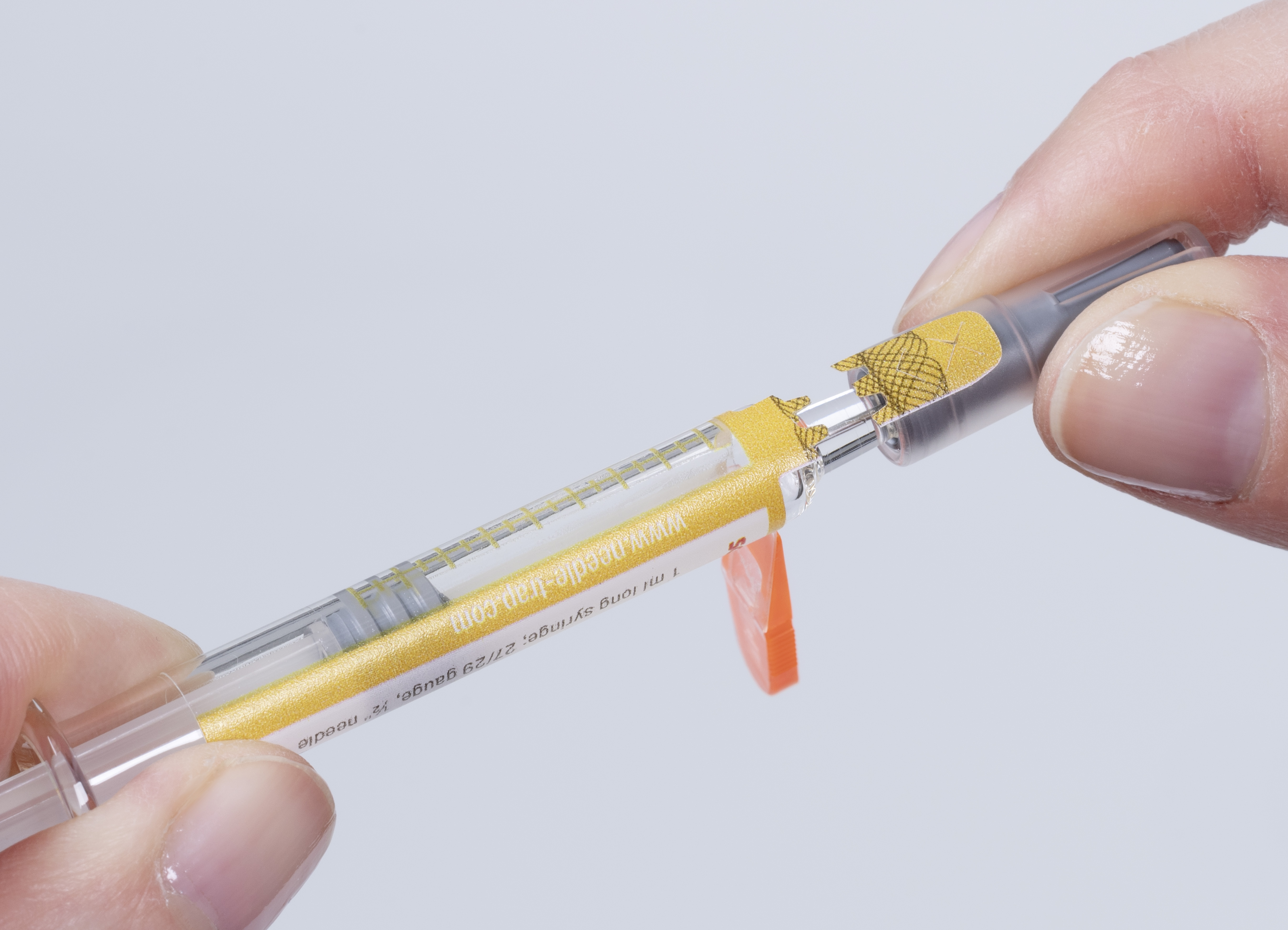
Due to its integrated plastic trap, the Needle-Trap needle protection label poses special challenges to a first-opening indication feature. In addition to smooth processing in pharmaceutical manufacturing operations, ease of use by healthcare staff also required consideration.
Schreiner MediPharm designed for its Needle-Trap solution a novel closure seal with a tab ending on the syringe cap. Before injection, the needle trap is first folded sideways, as usual. While the cap is being pulled off, a perforation automatically activates the label-integrated seal, which irreversibly indicates the label’s initial opening. Special security cuts prevent undetected removal of the seal. For authenticity verification, overt authentication features such as a guilloche pattern or covert security features can be added.
Needle-Trap’s new feature provides pharmaceutical manufacturers with a multifunctional and cost-efficient solution, combining protection of healthcare staff against needlestick injuries with first-opening indication that ensures unit-level prefilled syringe integrity. Healthcare staff benefit from efficient and reliable needle protection as well as convenient first-opening indication that is irreversible and detectable at first glance, enhancing both product and patient safety. In addition, the new Needle-Trap version can be easily integrated into existing pharmaceutical manufacturing processes.
For more information, visit our website at www.schreiner-medipharm.com