HERMA Label Paper Suited for Pharma Uses
- Published: March 12, 2015
FILDERSTADT, GERMANY | HERMA has developed the new HERMAlaser sheet 50 label paper, described as a lightweight and supple material that ensures the permanent, safe labeling of surfaces with small radii. 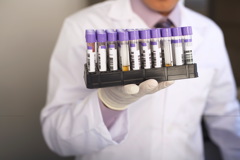
It is said to be suited for use in pharmaceutical applications, e.g., in hospitals or laboratories. “The new material’s low weight minimizes the stiffness and thereby the ‘restoring force’ of the labels,” says Ralf Drache, head of sales at HERMA. “The latter is especially noticeable when labeling delicate objects such as ampoules or syringes. But in such cases, good adhesion is essential—accidentally detached labels might have serious consequences.”
Company says the combination with its permanent adhesive 62Z ensures optimal results. The surface of HERMAlaser sheet 50 is designed for excellent printability and toner keying with all xerographic printing processes. The label sheets are therefore suitable for desktop laser printers, copiers, and many inkjet printers. The paper is approved according to the BfR recommendation XXXVI (Bundesinstitut für Risikobewertung, Federal Institute for Risk Assessment). It can therefore be safely used for food packaging and can be in direct contact with dry and fatty foodstuffs. The adhesive 62Z also features an approval for dry, moist, and fatty foodstuffs. Additionally, it can also be used for the direct labeling of blood bags that are used to store and transport blood donations and require especially reliable identification.